Water Softener and Demineralization
 Introduction
Introduction
The removal of impurities, such as calcium, magnesium, iron and silica which can cause scale, is known as water softening or demineralization. Common treatment methods to remove these impurities include lime softening, sodium cycle cation exchange (often called sodium zeolite softening), reverse osmosis, electrodialysis, and ion ex-change demineralization. Which treatment is most appropriate depends on the water supply quality, the purity requirements of the boiler, and to some extent – the budget.
Water Hardness is measured in grains per gallon or ppm. The conversion is 17.1 ppm = 1 grain
One cubic foot of softener resin is typically good for 30,000 grains in exchange. Softeners are typically set to regenerate once the resin is 90% exhausted. Regeneration is accomplished with a variety of chemicals for various purposes, but is commonly simple table salt brine, NaCl. The last part of the regeneration cycle is a fresh water flush to prevent salt from entering the boiler.
Operations
Quick Lime and Clarifiers
Quick or slaked lime added to hard water, reacts with the calcium, magnesium and, to some extent, the silica in the water to form a solid precipitate. The process typically takes place in a clarifier. The lime is added to the “rapid mix zone”, where it reacts with some of the calcium, magnesium and silica. The combined precipitate is removed from the bottom of the clarifier and the treated water is now softer than the untreated inlet water but still unsuitable for the boiler.
Lime softening treatment is followed by either sodium cycle cation exchange or ion ex-change demineralization. Cation exchange is usually picked for lower pressure boilers (450 psig) and demineralization for higher pressure boilers (above 600 psig).
Ion exchange is just what it implies: a process that exchanges one type of ion (charged particle) for another. Many troublesome impurities in supply water are ions, making this process extremely important in boiler water treatment. Ion exchange takes place in a closed vessel which is partially filled with an ion exchange resin. The resin is an insoluble, plastic-like material capable of exchanging one ion for another. There are two types: cation and anion resins. Each is capable of exchanging one or the other types of ions.
Cation = positively charged Ions
Anion = negatively charged Ions
Another method of ion exchange involves a sodium exchange softener, where hard water enters the unit and the calcium and magnesium are exchanged for sodium. The treated water will normally have most of the hardness removed, but will still contain other impurities. This method is suitable only for low pressure boilers.
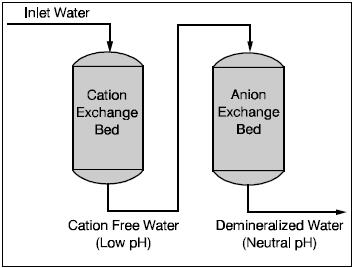
If very pure water is required, for high pressure boilers for example, then demineralization is required. A demineralizer contains one or more cation exchange beds, followed by one or more anion exchange beds.
In the demineralizer, water is treated in two steps. First, it is passed through the cation exchange bed, where the cations (calcium, magnesium and sodium) are exchanged for hydrogen ions. The treated water is now free of cations but is too acidic and cannot yet be used in the boiler. In the second step the water passes through the anion exchange bed where the anions (sulfate, chloride, carbonate and silica) are ex-changed for hydroxide ions. The hydrogen and hydroxide ions react to form water, now suitable for use in the boiler. A third ion exchange could be used to control alkalinity.
For higher purity water, more elaborate systems are employed, but the basic principle remains the same.
Ion exchange resins have a limited capacity and will eventually become exhausted. They can be regenerated however; sodium cycle cation exchange beds are regenerated with salt brine, cation exchange beds are regenerated with hydrochloric or sulfuric acid and the anion exchange beds become regenerated with caustic soda. Salt brine regeneration is followed by a fresh water rinse to assure that no salt enters the boiler.
Dealkalizers reduce the alkalinity of softened water through a chloride anion exchange process. Softened water is passed through the anion exchange resin where bicarbonate, carbonate, sulfate and nitrate ions are exchanged for chloride ions. The anion exchange resin is regenerated by salt (NaCl) and softened water. Some dealkalizers add a small amount of caustic during the regeneration cycle to increase capacity and provide a slightly elevated pH level.
The primary benefit of using dealkalized water is the prevention of CO2 generation inside of the boiler. CO2 leaves the boiler with the steam and can form carbonic acid in the condensate, leading to the primary cause of condensate system corrosion.
Other Technologies
Other technology is sometimes employed to remove undesirable impurities from the water supply, including reverse osmosis, electrodialysis, and electrodialysis with current reversal. These are all known as membrane processes. Reverse osmosis uses semipermeable membranes that let water through but block the passage of salts. In the case of electrodialysis, the salts dissolved in the water are forced to move through cation-selective and anion-selective membranes, removing the ion concentration.
More on Reverse Osmosis
More Information
Source: CIBO Energy Efficiency Guide 1997; edited by Bob Fegan with minor contributions from other sources 1-2005; image of water softener from web site http://www.heatandpowerproducts.com/steam.html 3/2005;