pH Treatment
Introduction
What is pH?
Acidic and basic (alkaline) are two extremes that describe chemicals, just like hot and cold are two extremes that describe temperature. Mixing acids and bases (alkaline) can cancel out their extreme effects, much like mixing hot and cold water can even out the water temperature. A substance that is neither acidic nor basic (alkaline) is neutral.
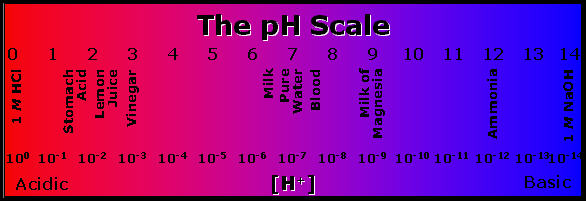
The pH scale measures how acidic or basic (alkaline) a substance is. It ranges from 0 to 14. A pH of 7 is neutral. A pH less than 7 is acidic, and a pH greater than 7 is basic (alkaline). Each whole pH value below 7 is ten times more acidic than the next higher value. For example, a pH of 4 is ten times more acidic than a pH of 5 and 100 times (10 times 10) more acidic than a pH of 6. The same holds true for pH values above 7, each of which is ten times more alkaline (another way to say basic) than the next lower whole value. For example, a pH of 10 is ten times more alkaline than a pH of 9.
Pure water is neutral, with a pH of 7.0. When chemicals are mixed with water, the mixture can become either acidic or basic (alkaline). Vinegar and lemon juice are acidic substances, while laundry detergents and ammonia are basic (alkaline).
Chemicals that are very basic or very acidic are called “reactive.” These chemicals can cause severe burns. Automobile battery acid is an acidic chemical that is reactive. Automobile batteries contain a stronger form of some of the same acid that is in acid rain. Household drain cleaners often contain lye, a very alkaline chemical that is reactive.
An Acid is a substance that produces H3O+ (H+) when it is dissolved in water. It is a proton donor and an electron pair acceptor or a species that donates protons. For example: HCl, NH4, AlCl3.
A Base is a substance that produces an OH- when it is dissolved in water (Arrhenius). A proton acceptor (Brønsted), or a electron donor. For example: NaOH, KOH, CH3NH2.
H2O can act as an acid or a base because it auto-ionizes itself, meaning it gives protons back and forth within itself, thus acting as both an acid and a base.
Boiler pH
Natural water is usually between 6.5 and 7.5 pH. A common recommendation is to maintain boiler water at 8.5 pH.
Acidic water is corrosive. Alkalinic water is more prone to scaling.
Alkalinity is a measure of the bicarbonate (HCO3), carbonate (CO3) and hydroxyl (OH) ions in the water. pH and alkalinity ratings are NOT the same and are NOT proportional. pH is rated on the Scale and alkalinity is measured in parts per million (ppm). A typically recommended alkalinity rating is 140 – 700 ppm for boilers operating below 300 psi.
Controlling pH
pH is controlled by either removing water impurities or adding other chemicals to neutralize the condition. For example, Caustic Soda, an alkaline, is added to neutralize CO3, carbonic acid.
pH Related Corrosion
Acid Attack
When the boiler water pH drops below about 8.5, a corrosion called acid attack can occur. The effect exhibits rough pitted surfaces. The presence of iron oxide deposits on boiler surfaces can encourage this kind of corrosion. A low boilerwater pH is usually caused by contamination of the boiler feedwater, from sources such as hydrochloric or sulfuric acid from leaks in demineralizers and condenser leaks of cooling tower water. Contamination can also occur from process leaks of acid or acid-forming materials into the return condensate system.
Caustic Attack
Caustic attack on boilers is a localized attack due to extremely high pH (12.9 +). It can take two forms: caustic gouging or caustic cracking, also called caustic embrittlement. Caustic attack or caustic corrosion, is often encountered in phosphate treated boilers in which deposits of phosphates or other ‘scale’ occur in high heat transfer areas. Boiler water can permeate the porous deposit resulting in localize corrosion. When it is coupled with significant heat flux, concentration of the boiler water occurs rapidly speeding the corrosion.
The corrosion action is a result of the formation of caustic-ferritic compounds through the dissolving of the protective magnetite film. Once the process begins, the iron in contact with the boiler water will attempt to restore the protective magnetite film. Caustic corrosion (typically in the form of gouging) continues until the deposit is removed or the caustic concentration is reduced to normal.
Caustic soda (NaOH) is the only normal boiler water constituent that has high solubility and does not crystallize under typical boiler conditions. Its caustic concentration can be as high as 10,000-100,000 ppm.
Careful control of boiler water chemistry can prevent caustic gouging. If the “free hydroxide alkalinity”
is set too high or uncontrolled, then caustic gouging may result. Prevention of porous deposit formation (such as iron oxide) eliminates a place for caustic gouging to occur.
