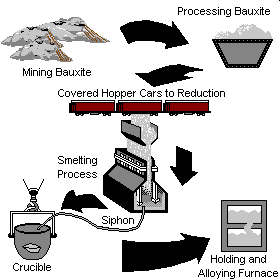
Mining and Primary Processing: Process Description
 Energy Consumption
Energy Consumption
Process Description
Aluminum is derived from the naturally occurring ore called Bauxite. Bauxite is refined into alumina which is electrochemically reduced into aluminum in reduction cells or pots (Hall-Herault process). The molten aluminum is then either cast into ingots, bars, rolled into sheets, plates or foil, or drawn into rod. These intermediate shapes are then shipped to processing plants which shape the aluminum into consumer products. Click on a process step below for more information.
Bauxite Mining
Bauxite consists of 45-60% aluminum oxide, 12-30% water, and various other impurities. Bauxite is typically mined in open-pits and either processed into alumina near the mining operation, or shipped to smelting markets around the world for processing. Less than 1% of aluminum in the U.S. comes from domestic bauxite. Major bauxite producing countries include Australia, Guinea, Brazil, Jamaica, and the former republics of the U.S.S.R.

Courtesy of the Aluminum Association
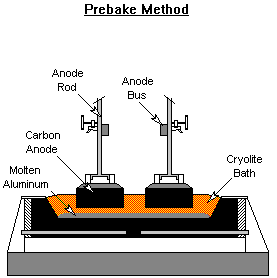
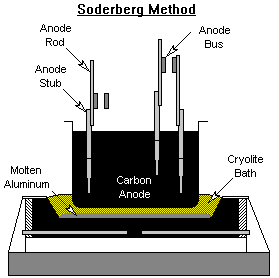
Anode Baking
Two types of carbon anodes are used in the cells. The Soderberg process bakes the carbon into anodes in the Hall-Heroult reduction cell. The heat liberated from the alumina reduction slowly bakes the carbon anode as it moves down the cell. Carbon anode paste is continuously replenished at the top of the cell. The prebake method forms the carbon anodes in ovens outside the reduction cell. The fully formed anodes are inserted into the molten aluminum in the smelting pot to reduce the alumina into aluminum. Anode baking is typically carried out in gas fired ovens.
Bauxite Refining
Bauxite is converted to alumina using the Bayer process. Bauxite is combined with caustic soda, lime, and steam to produce a sodium aluminate liquor. Impurities are filtered or settled out of the liquor and alumina hydrate is precipitated out of the mixture. The aluminu hydrate is calcined to remove moisture and drive off the bonded water. The resulting alumina is ready for smelting into aluminum. Calcining is carried out in large gas fired rotary kilns, representing a significant process gas load. However, only about half of the alumina supplies in the U.S. are refined domestically.

“Industrial Energy Efficiency”, Office of Technology Assessment, August 1993
Molten Aluminum Handling
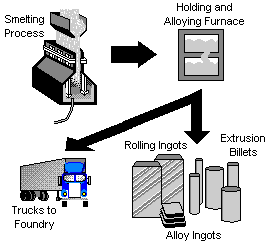
The molten aluminum is siphoned from the smelting pot and is either formed into ingots, transferred to an on-site processing plant where rolling ingots, extrusion billets, sheets, or rod is formed. In some cases, the molten aluminum is trucked to nearby foundries for casting.
Aluminum Smelting
Alumina is electrolytically reduced into molten aluminum. This reaction occurs in Hall-Heroult reduction cells (called cells or pots) where the bound oxygen in the alumina reacts with carbon electrodes to form carbon dioxide gas and aluminum. Each ton of aluminum requires 0.4-0.5 tons of carbon anodes.

Courtesy of Washington CEO Magazine